Mumbai, Maharashtra Feb 22, 2024 (Issuewire.com) - Company Contact:
Mr. Manoj Mishra/ Uday S. Chile
+91-9823163973/+91-9324403484
Brassica Pharma Pvt. Ltd. is voluntarily recalling Eye Ointment products listed in the table below with expiration dates ranging from February 2024 to September 2025. The products are being recalled due to a lack of sterility assurance at the facility noted during an inspection conducted by the Food and Drug Administration (FDA).
Risk Statement: For those patients who use these products, there is a potential risk of eye infections or related harm. These products are intended to be sterile. Ophthalmic drug products pose a potentially heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defenses. To date, Brassica Pharma Pvt. Ltd. has not received any reports of adverse events upto 16th February 2024 related to this recall.
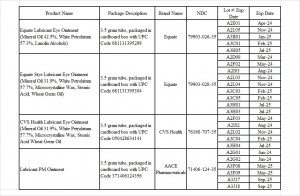
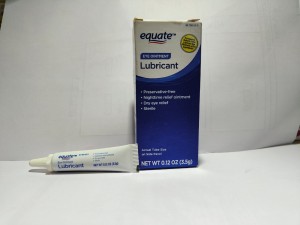
Product Name: Equate Lubricant Eye Ointment (Mineral Oil 42.5%, White Petrolatum 57.3%, Lanolin Alcohols)
Package Description: 3.5-gram tube, packaged in a cardboard box with UPC Code 681131395298
Brand Name: Equate
NDC: 79903-026-35
Lot #/ Exp Date: A2E01- Apr-24; A2L05-Nov-24; A3B01- Jan-25; A3C01- Feb-25; A3H05- Jul-25
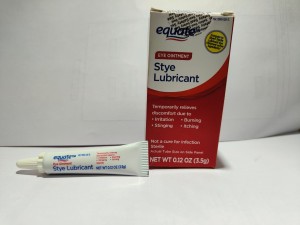
Product Name: Equate Stye Lubricant Eye Ointment (Mineral Oil 31.9%, White Petrolatum 57.7%, Microcrystalline Wax, Stearic Acid, Wheat Germ Oil)
Package Description: 3.5-gram tube, packaged in a cardboard box with UPC Code 681131395304
Brand Name: Equate
NDC: 79903-028-35
Lot #/ Exp Date: A2D08-Mar-24; A2F02-May-24; A2I03Aug-24; A2L03-Nov-24; A2L04-Nov-24; A3C03-Feb-25; A3C05-Feb -25; A3H01-Jul-25; A3H03-Jul-25
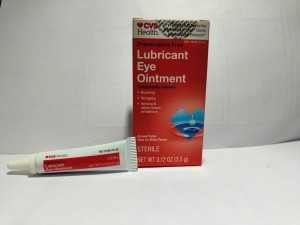
Product Name: CVS Health Lubricant Eye Ointment (Mineral Oil 31.9%, White Petrolatum 57.7%, Microcrystalline Wax, Stearic Acid Wheat Germ Oil
Package Description: 3.5-gram tube, packaged in a cardboard box with UPC Code 050428634141
Brand Name: CVS Health
NDC: 76168-707-35
Lot #/ Exp Date:
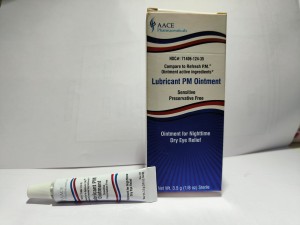
Product Name: Lubricant PM Ointment
Package Description: 3.5-gram tube, packaged in a cardboard box with UPC Code 371406124356
Brand Name: AACE Pharmaceuticals
NDC: 71406-124-35
Lot #/ Exp Date: A2F03-May-24; A2I02-Aug-24; A2L02-Nov-24; A3C04-Feb-25; A3H04-Jul-25
These products were distributed nationwide to wholesalers, retailers, and via the product distributor, Walmart, CVS, and AACE Pharmaceuticals Inc.
Brassica Pharma Pvt. Ltd. Is notifying its distributors AACE Pharmaceuticals Inc. and its retailers Walmart and CVS. These distributors shall further notify the wholesalers and retailers via mail of this voluntary recall and arrange for the return of all impacted products listed above. Consumers, distributors, and retailers that have any product that is being recalled should cease distribution of the product. Consumers should stop using the recalled Eye Ointment and may return any of the above-listed products to the place of purchase.
Consumers with questions regarding this recall can contact Brassica Pharma Pvt. Ltd. at +1 833-225-9564 or info@brassicapharma.com. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download the form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Media Contact
Brassica Pharma Pvt Ltd *****@brassicapharma.com +91 (0) 22 2870 4581 701/702, 7th floor, vastubh, carter road no.1, Borivali East, Mumbai Suburban, Maharashtra, 400066 https://www.brassicapharma.com